
If this is the first set of questions you have done, please read the introductory page before you start. The easiest thing to add is usually dilute sulphuric acid - but any other soluble sulphate would do. If you add colourless potassium iodide solution (or any other source of iodide ions in solution) to a solution of lead(II) nitrate, a bright yellow precipitate of lead(II) iodide is produced.Īdding a source of aqueous sulphate ions to a solution of lead(II) nitrate results in a white precipitate of lead(II) sulphate.

Complex ions like PbCl 4 2- are produced, and these are soluble in water. Note: If you add concentrated hydrochloric acid to excess, the lead(II) chloride precipitate will dissolve again. You could use things like sodium chloride solution to provide the chloride ions, but it is usually easier just to add some dilute hydrochloric acid. Translations Context sentences Monolingual examples Synonyms Conjugation.
#Precipitate examples free
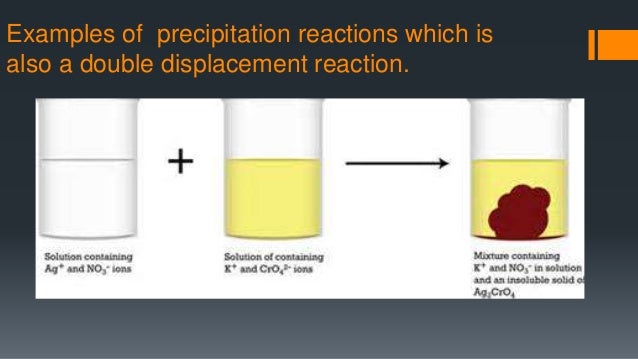
Lead(II) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(II) nitrate solution. Translation for precipitate in the free English-Czech dictionary and many. I am using these particular versions of the equations to keep them in line with the corresponding reaction between lead(II) oxide and sodium hydroxide solution on the oxides of Group 4 page - also a simplification! You will get complexes formed involving hydroxide ions, but the formulae of these aren't very clear-cut. Note: These equations are simplifications. If more sodium hydroxide solution is added, the precipitate redissolves to give a colourless solution which might be called sodium plumbate(II) solution - but could be called by a lot of alternative names depending on exactly how the formula is written! If a little sodium hydroxide solution is added to colourless lead(II) nitrate solution, a white precipitate of lead(II) hydroxide is produced. Raindrops form around microscopic cloud condensation nuclei, such as a particle of dust or a molecule of pollution. Rain Rain is precipitation that falls to the surface of the Earth as water droplets. It describes the reactions to form lead(II) hydroxide, lead(II) chloride, lead(II) iodide and lead(II) sulphate.īecause of the insolubility of so many lead(II) compounds, the usual source of lead(II) ions in solution is lead(II) nitrate solution - and that will be assumed in all the following examples. The most common types of precipitation are rain, hail, and snow. Example 1: Explaining Why Ashless Filter Paper Is Used in Gravimetric Analysis Because it filters the precipitate from the solution efficiently Because we. This page looks at the formation of some insoluble lead(II) compounds from aqueous lead(II) ions using precipitation reactions.


 0 kommentar(er)
0 kommentar(er)
